Enhancing Electrode Surface Area
Platinum electrodes
Platinum electrodes make use of a specialized surface treatment the results in a very high surface area electrode. Electrodeposition of platinum black from chloroplatinic acid produces a surface covered with platinum black – a from of very finely divided, porous platinum. These platinized platinum electrodes have a BET surface area 400-500 times their macroscopic geometrical area1), and can have three orders of magnitude greater capacitance than shiny platinum electrodes2).
Studies3) of activation methods of platinum electrodes show that CPE-P4) values increase with increasing salt concentration in the electrolyte. Capacitance also increases with increasing salt concentration, roughly as the log of concentration. Effective electrodes require activation, consisting of 50 cycles of cyclic voltametry with a platinum counter electrode, each cycle between appx. +/- 1.2V, and about 1/2 hour per cycle. CV curves stabilized only after many cycles. There is significant drift in capacitance over time, requiring periodic (perhaps weekly) reactivation.
The frequency dependence of capacitance on solid electrodes is due to the interaction of atomic scale inhomogeneities with the electrolyte rather than to the geometry aspect of roughness5).
Graphite electrodes
The following photo shows the two paraxial graphite electrodes discussed on the page, Cell Constant of Parallel Wire Electrodes.
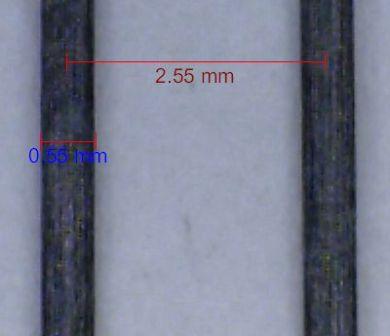
A close-up view of one of the electrodes shows deep grooves and high surface irregularity in the extruded polymer-graphite-carbon rods (1pixel =0.75 μm):

Carbon and pyrolytic carbon
Graphite
Glassy carbon
Glassy carbon is used in electrochemistry owing to its high electrical conductivity, immunity to surface contamination, and very good corrosion resistance.
Glassy carbon has no open porosity – so it probably has much lower surface area than graphite.
Glassy carbon can be oxidatively activated to increase its surface area three to five orders of magnitude, as measured by an increase in capacitance6) and BET surface area7).
Suppliers
- Pine Intsturments carbon screen printed electrodes
- Pine Intsturments Glassy carbon electrodes
- Pine Intsturments Pyrolytic carbon electrodes
About glassy carbon electrodes:
Stainless steel
Type 304 stainless steel electrodes may provide electrodes of sufficient surface area, particularly if their microscopic area is enhanced by treatment with abrasives or electrolytic etching.
Here is a photo of a 0.050" (1.27mm) 304 stainless steel tube, closed on the end, and a close-up photo of the same tube, showing about ¼ of the diameter (1pixel =0.75 μm).

It is demonstrated that glassy carbon powder can be thermochemically activated. During activation, a film with open pores is created on the glassy carbon particles. This film has a large internal surface area, which is accessible to liquids and gases. A simple model for the evolution of the internal surface area in glassy carbon powder during thermochemical gas-phase oxidation is also presented and compared with experimental data. Experimental results are in qualitative agreement with the model.We found that a sharp particle size distribution is desirable with regard to potential technical applications.
